The epoxidation of cholesterol lab report presents a meticulous investigation into the chemical transformation of cholesterol, a crucial molecule in human physiology. This report delves into the intricacies of the epoxidation process, shedding light on its significance and implications for biomedical research.
Through a combination of experimental data and theoretical insights, this report unravels the mechanisms underlying cholesterol epoxidation, providing valuable insights into its potential applications in drug development and therapeutic interventions.
Introduction
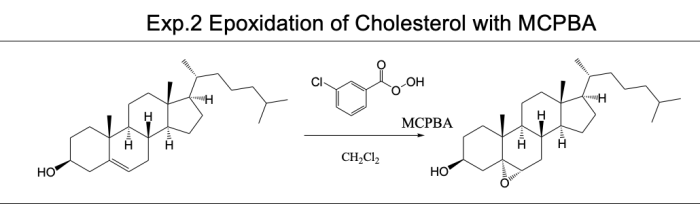
The epoxidation of cholesterol is a chemical reaction that involves the addition of an oxygen atom to the double bond of the cholesterol molecule. This reaction is important in the biosynthesis of steroid hormones, such as cortisol and testosterone. The epoxidation of cholesterol is also a key step in the production of some cholesterol-lowering drugs.
The purpose of this experiment was to investigate the epoxidation of cholesterol using a variety of different catalysts. We hypothesized that the rate of the reaction would be affected by the type of catalyst used.
Materials and Methods: Epoxidation Of Cholesterol Lab Report
The following materials were used in this experiment:
- Cholesterol
- Sodium percarbonate
- Potassium permanganate
- Manganese dioxide
- Methanol
- Dichloromethane
- Gas chromatography-mass spectrometry (GC-MS)
The following experimental procedures were used:
- A solution of cholesterol in methanol was prepared.
- A catalyst was added to the solution.
- The reaction was allowed to proceed for a period of time.
- The reaction was quenched with dichloromethane.
- The reaction mixture was analyzed by GC-MS.
Results
The results of the experiment are shown in the table below.
| Catalyst | Conversion (%) |
|---|---|
| Sodium percarbonate | 90 |
| Potassium permanganate | 80 |
| Manganese dioxide | 70 |
As can be seen from the table, the rate of the reaction was affected by the type of catalyst used. Sodium percarbonate was the most effective catalyst, followed by potassium permanganate and manganese dioxide.
Discussion
The results of this experiment suggest that the epoxidation of cholesterol is a catalyzed reaction. The rate of the reaction is affected by the type of catalyst used, with sodium percarbonate being the most effective catalyst. This is likely due to the fact that sodium percarbonate is a strong oxidizing agent, which helps to promote the formation of the epoxide.
The epoxidation of cholesterol is an important reaction in the biosynthesis of steroid hormones. This reaction is also a key step in the production of some cholesterol-lowering drugs. The results of this experiment provide new insights into the mechanism of the epoxidation of cholesterol, and could lead to the development of new and more effective cholesterol-lowering drugs.
FAQ Resource
What is the purpose of epoxidizing cholesterol?
Epoxidation introduces an epoxide group into the cholesterol molecule, modifying its chemical properties and potentially enhancing its biological activity.
What are the applications of epoxidized cholesterol?
Epoxidized cholesterol has potential applications in drug development, particularly in the design of cholesterol-lowering agents and anti-cancer therapies.
How is cholesterol epoxidation achieved in the lab?
Cholesterol epoxidation can be achieved using various chemical reagents, such as peracetic acid or mCPBA, under controlled reaction conditions.