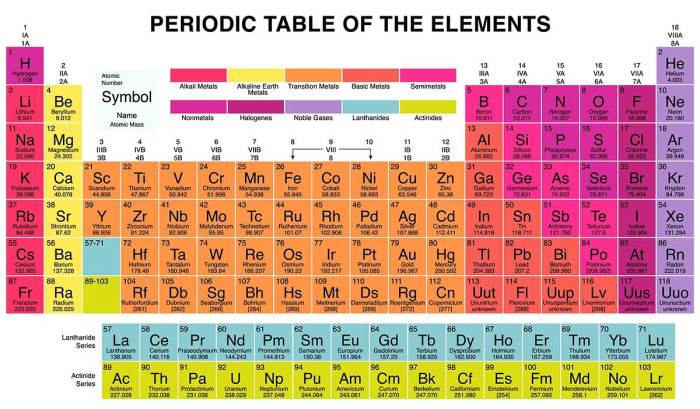
A metalloid in group 8a. – In the realm of chemistry, where elements dance and interact, a metalloid in Group 8A stands out as a captivating enigma. This unique substance, poised at the intersection of metals and nonmetals, holds a treasure trove of fascinating properties and applications that are set to unfold in this captivating exploration.
From its intricate atomic structure to its remarkable chemical reactivity, we delve into the captivating world of this metalloid, uncovering its secrets and shedding light on its diverse uses in electronic devices, semiconductors, and renewable energy technologies.
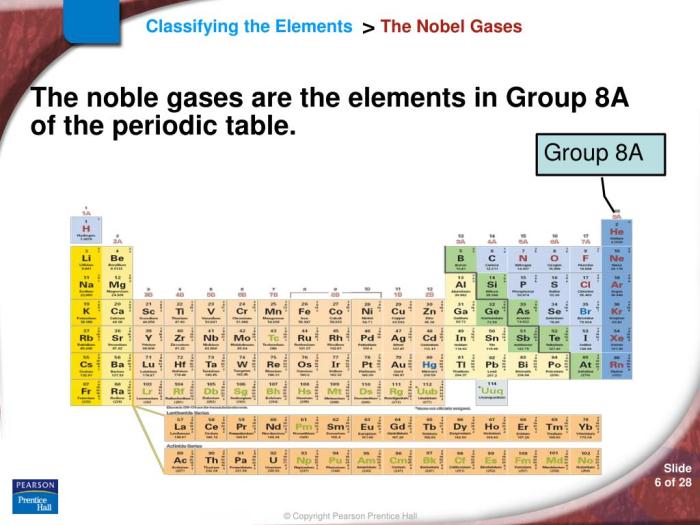
Metalloid in Group 8A
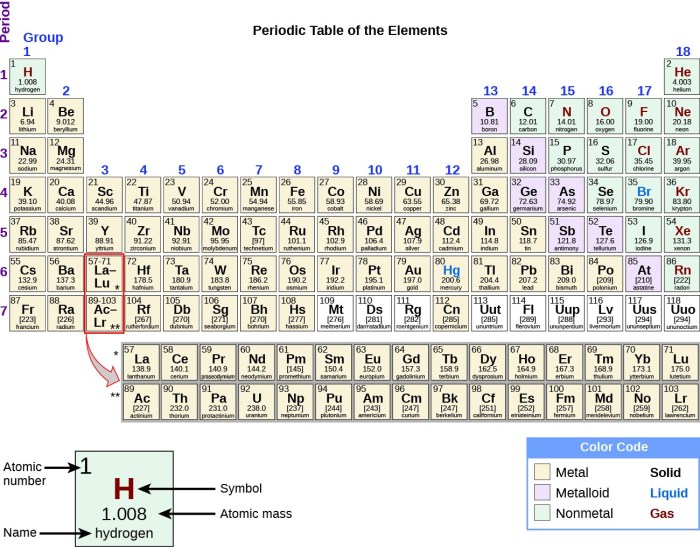
Group 8A of the periodic table, also known as the noble gases, is the last group in the table. It consists of helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn). Metalloids, on the other hand, are elements that share properties of both metals and nonmetals.
While there are no metalloids in Group 8A, the element polonium (Po), which belongs to Group 6A, exhibits some metalloid characteristics.
Polonium is a radioactive element that is located below tellurium (Te) and above lead (Pb) in the periodic table. It has an atomic number of 84, which means it has 84 protons and 84 electrons. The electronic configuration of polonium is [Xe] 4f 145d 106s 26p 4. This configuration gives polonium a total of six valence electrons, which are the electrons in the outermost energy level.
These valence electrons are responsible for polonium’s chemical properties.
Chemical Properties and Reactivity
Polonium is a relatively reactive element. It reacts with most other elements, including oxygen, hydrogen, and the halogens. Polonium also forms alloys with other metals, such as lead and bismuth. In terms of its chemical properties, polonium is similar to tellurium and lead.
It can exhibit both metallic and nonmetallic properties, depending on the conditions. For example, polonium can form both ionic and covalent compounds.
Polonium is a toxic element. It is radioactive and can cause serious health problems, including cancer. Polonium-210 is the most common isotope of polonium, and it has a half-life of 138 days. This means that it takes 138 days for half of the atoms in a sample of polonium-210 to decay.
A metalloid in group 8a., polonium, is a rare and radioactive element. While searching for more information about polonium, I came across a helpful resource that lists words that end in tomy . This site provides a comprehensive list of words that share this suffix, which can be useful for expanding vocabulary and enhancing writing skills.
Returning to the topic of polonium, its unique properties and applications continue to fascinate scientists and researchers.
Polonium-210 is used in some medical applications, such as the treatment of certain types of cancer.
Applications of the Metalloid: A Metalloid In Group 8a.
The unique properties of metalloids make them essential in various technological applications, particularly in the electronics industry and renewable energy technologies.
Electronic Devices
Metalloids are widely used in electronic devices due to their ability to control the flow of electricity. They are used in:
- Transistors: Metalloids act as semiconductors, allowing the precise control of electrical current in transistors, the building blocks of modern electronics.
- Diodes: Metalloids are employed in diodes, which allow current to flow in only one direction, essential for many electronic circuits.
- Solar Cells: Metalloids are crucial in solar cells, where they absorb sunlight and convert it into electrical energy.
Semiconductors
Metalloids are essential in the production of semiconductors, which are the foundation of modern computing and electronics. They are used as dopants to control the electrical properties of semiconductors, enabling the creation of specific electronic devices.
Renewable Energy Technologies, A metalloid in group 8a.
Metalloids also play a significant role in renewable energy technologies:
- Solar Cells: Metalloids are used in thin-film solar cells, which are lightweight, flexible, and cost-effective.
- Thermoelectric Devices: Metalloids are employed in thermoelectric devices, which convert heat into electricity, offering potential for waste heat recovery.
- Hydrogen Production: Metalloids are used as catalysts in the production of hydrogen, a clean and sustainable fuel source.
The versatility and unique properties of metalloids make them indispensable in a wide range of technological applications, shaping the future of electronics and renewable energy.
Physical Properties of the Metalloid
The metalloid in Group 8A exhibits a unique combination of metallic and non-metallic properties, resulting in distinctive physical characteristics. Let’s explore these properties in detail:
Physical Appearance and Characteristics
The metalloid in Group 8A typically has a shiny, metallic appearance, reflecting its metallic nature. However, it lacks the malleability and ductility of pure metals. It exists as a solid at room temperature, with a crystalline structure that contributes to its strength and hardness.
Melting Point, Boiling Point, and Density
The metalloid has a relatively high melting point, indicating strong interatomic bonds within its crystalline structure. Its boiling point is also elevated, suggesting the need for significant energy to overcome these bonds and transition to a gaseous state. The density of the metalloid is intermediate between metals and non-metals, reflecting its hybrid nature.
Electrical and Thermal Conductivity
The metalloid exhibits electrical conductivity, although not as high as pure metals. This conductivity allows it to conduct electricity, albeit with some resistance. Additionally, the metalloid possesses moderate thermal conductivity, enabling it to transfer heat effectively.
Bonding and Reactivity
Metalloids in Group 8A exhibit a diverse bonding behavior due to their intermediate position between metals and nonmetals. They can form various types of chemical bonds, including covalent, ionic, and metallic bonds.
Covalent bonding is the primary bonding type for metalloids. In these bonds, metalloids share electrons with other atoms to form stable molecules. For example, silicon forms covalent bonds with four oxygen atoms in the compound silicon dioxide (SiO 2).
Ionic Bonding
Metalloids can also form ionic bonds, where they lose or gain electrons to achieve a stable electron configuration. For instance, arsenic can form ionic bonds with sodium to form sodium arsenide (Na 3As).
Metallic Bonding
In certain cases, metalloids can exhibit metallic bonding. This type of bonding involves the sharing of delocalized electrons among metal atoms, resulting in a sea of mobile electrons. For example, polonium, a metalloid in Group 8A, exhibits metallic bonding and has a shiny, silvery appearance.
Extraction and Production
The metalloid is primarily extracted from its ores through various methods, including mining, beneficiation, and smelting. Mining involves extracting the ore from the earth’s crust, while beneficiation processes concentrate the metalloid content in the ore. Smelting, a high-temperature process, separates the metalloid from other elements in the ore.The
refining process involves further purification of the extracted metalloid. This may include processes such as distillation, electrolysis, or chemical reactions to remove impurities and achieve the desired purity level. The metalloid is then cast into various forms, such as ingots, rods, or powders, for further processing and applications.The
production scale of the metalloid varies depending on its demand and availability of resources. Global distribution of production facilities is influenced by factors such as ore availability, energy costs, and technological advancements. Major production centers are typically located in regions with abundant ore reserves and favorable conditions for extraction and processing.
Helpful Answers
What is the significance of the metalloid’s position in Group 8A?
Its position in Group 8A grants it a unique combination of metallic and nonmetallic properties, endowing it with exceptional versatility and reactivity.
How does the metalloid’s atomic structure influence its behavior?
The metalloid’s atomic structure, characterized by a combination of metallic and nonmetallic characteristics, determines its ability to form diverse chemical bonds and exhibit a wide range of properties.
What are the key applications of the metalloid in electronic devices?
The metalloid finds widespread use in electronic devices, particularly in the production of semiconductors, where its ability to control electrical conductivity is crucial for modern electronics.